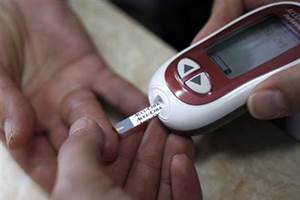
Glucometer, the instant handheld device which tells you the level of sugar in your blood, costs around Rs 2000 to Rs 3000 and the strips associated with it costs around Rs 15 – 20 each. According to a The Indian Express report, almost 9 percent of the Indian population suffer from diabetes, and in that case, the cost involved is a huge burden. With an aim to find a solution to this issue, Indian Council of Medical Research (ICMR) had asked technical and medical institutes in the country to bring ideas to manufacture low-cost devices. After funding 20 projects, three of them were selected to be scaled up, and BITS Pilani’s idea was the first to make it.
But even after PM Narendra Modi’s emphasis and the high-cost burden, the market has yet not seen the low-cost glucometer and glucose testing strips. What is even more appalling is the fact that both ICMR and the company called Soothe Healthcare which was reportedly supposed to manufacture the device, are playing the blame game. BITS Pilani had developed the device but for 18 months it has remained on the drawing board since Soothe Healthcare was handed over the manufacturing and marketing rights. Former health minister Ghulam Nabi Azad had taken the initiative and emphasised on the need for India to work on Glucometers.
According to ICMR the medical company Soothe is very new in the field and-and that makes it difficult to get the required licences and that is what they have been doing for the most part in two years. Though ICMR claimed that the handover happened in February 2015, and it looked like the device would come to the market within six months. Soothe Healthcare, on the other hand, claims that invalidated technology was handed over to them a full year after they signed a MoU and paid the council.
However, ICMR said that the technology is not exclusive and if a different company comes forward to manufacture and market then it will be shared. “The commercial partner was chosen based on expressions of interest received. We handed the technology over to Soothe in August 2015. Our assessment was the machine could be marketed for Rs 500 and the strips for about Rs 2. When we signed the MoU our understanding was that it would be in the market in six months. However that did not happen.” said Dr Chandra Shekhar, Scientist at ICMR told The Indian Express.
However, Sahil Dahiya, CEO of Soothe, told a different version to The Indian Express, “ICMR did not sign the MoU until February 2015 and after taking the money — the sum is confidential — did not give us the dossier for the technology for the full year. They had not even applied for a patent and did not pay BITS Pilani till about a month ago. My experience of working with ICMR has been very bad, they take days to move a file from one room to the next. It was not even validated, so we will have to test the prototypes on 2,000 people to know whether it even works.”
According to ICMR, there are two more technologies in the pipeline, one of which is in the validation stage. There is another low-cost technology which does not even require blood which ICMR expects to get in six weeks.