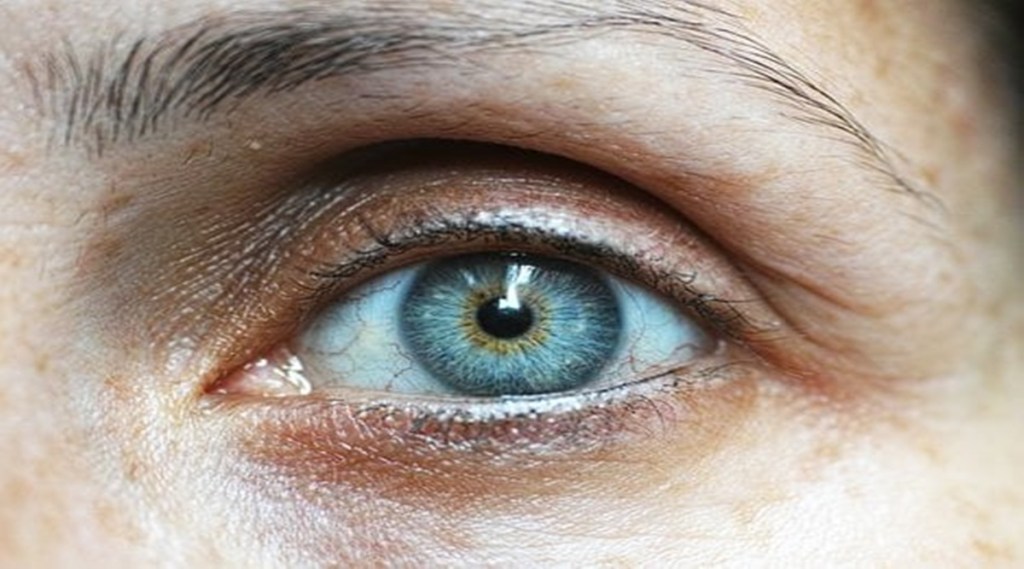
Roche on Thursday announced the results of its two global phase III studies which evaluated the bispecific antibody for the eye, Vabysmo. According to the pharma company, during the trial, the drug improved the vision of participants with macular edema due to branch and central retinal vein occlusion (BRVO and CRVO).
Retinal Vein Occlusion (RVO) is a blockage of the small veins that carry blood away from the retina. Studies point out that this vision-threatening condition affects 28 million people globally.
“Both studies met their primary endpoints, showing that people with macular edema due to BRVO and CRVO receiving Vabysmo injections every four weeks, for up to 24 weeks, achieved non-inferior visual acuity gains compared to those receiving aflibercept injections every four weeks,” the company stated on Thursday.
According to the company, Vabysmo also showed rapid drying of retinal fluid from baseline through week 24, as measured by a reduction in central subfield thickness.
“These encouraging data demonstrate that Vabysmo could potentially provide a new treatment option for people living with retinal vein occlusion, a serious retinal vascular condition that can lead to irreversible vision impairment or vision loss. Today’s results add to the extensive evidence supporting Vabysmo’s efficacy in treating multiple types of retinal conditions. We look forward to submitting these data to regulatory authorities,” said Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development in a statement.
In both studies, Vabysmo was generally well tolerated and the safety profile was consistent with previous trials, the company claims. Moreover, the detailed results will be presented at an upcoming medical meeting and submitted to regulatory authorities around the world.
“Vabysmo is uniquely engineered to target and inhibit two disease pathways linked to a number of vision-threatening retinal conditions by neutralising angiopoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A) to restore vascular stability. The level of Ang-2 is elevated in RVO and it is thought that increased Ang-2 expression drives disease progression,” the company stated.
Vabysmo is approved in more than 40 countries around the world, including the United States, Japan, the United Kingdom and the European Union, for people living with neovascular or ‘wet’ age-related macular degeneration (nAMD) and diabetic macular edema (DME).
The pharma major claims that Vabysmo is the only injectable eye medicine approved with phase III studies supporting treatment intervals of up to four months for people living with nAMD and DME.
Globally, more than 165,000 Vabysmo doses have been distributed for the treatment of these conditions to date, as per the company data.
ALSO READ | Roche’s SMA drug shows positive outcomes in largest trial ever