The new guidelines issued by the National Medical Commission (NMC) have sparked a row among doctors and health experts. According to guidelines, issued on August 2, it is now mandatory for doctors to prescribe generic drugs and they may be penalised or their license might be suspended if they fail to do so.
The National Medical Commission (NMC) in its ‘Regulations relating to Professional Conduct of Registered Medical Practitioners” also asked doctors to avoid prescribing branded generic drugs.
“Every Registered medical professional should prescribe drugs using generic names written legibly and prescribe drugs rationally, avoiding unnecessary medications and irrational fixed-dose combination tablets,” NMC stated in its guidelines.
The regulatory body of medical education and medical professionals also stated that doctors can “prescribe or supply drugs, remedies, or appliances as long as there is no exploitation of the patients.”
“Drugs prescribed by RMP or bought from the pharmacy for a patient should explicitly state the generic name of the drug,” added.
The new guidelines led to a massive uproar among doctors and many of them took to social media to voice their opinion. On Monday, Indian Medical Association (IMA) issued a statement strongly opposing the regulation.
“The notification is an injustice to doctors who always hold the interest of their patients as non-negotiable. If the government and NMC want all the doctors in the country to prescribe only generic drugs, they should simply order all pharmaceutical companies to manufacture all the drugs without brand names. Then no one has to write the brand name. Let the NMC/GOI ensure quality generic drugs or accept responsibility if patients fail to respond to prescribe generics,” the association said in a statement.
According to a report by Indian Express, IMA is planning to meet Union Health Minister Mansukh Mandaviya over the issue next week. IMA also stated that it urges the government to have ‘one drug, one quality, one price’ system whereby all brands should be either sold at the same price which should be controlled or banned, and only generics allowed while ensuring the highest quality of these drugs.
Generic VS Branded drugs
In its latest guidelines, NMC stated that as generic medicines are 30 to 80 percent cheaper than branded drugs, prescribing generic medicines may overtly bring down healthcare costs and improve access to quality care.
The regulatory body defines generic drug as “drug product that is comparable to brand/reference listed product in dosage in dosage form, strength, route of administration, quality and performance characteristics, and intended use.”
Meanwhile, a branded generic drug is one which has come off patent and is manufactured by drug companies and sold under different companies’ brand names.
“These drugs may be less costly than the branded patent version but costlier than the bulk manufactured generic version of the drug. There is less regulatory control over the prices of these “branded” generic drugs,” it stated.
According to experts, when a new drug is launched by a pharmaceutical company with a specific name and patent protection it is called a branded drug. These drugs are more expensive as they are newer, generally groundbreaking, and often for medical conditions that are difficult to treat. However, after a certain period of time when the patent expires, generic medicines can be launched for these branded drugs.
According to the United States Food and Drug Administration (USFDA), generic medicines work in the same way and provide the same clinical benefit and risks as their brand-name counterparts.
What do the new NMC guidelines say?
According to NMC, doctors can prescribe drugs with “generic”/“non-proprietary”/“pharmacological” names only. However, in the case of drugs with a narrow therapeutic index, biosimilars, and similar other exceptional cases, this practice can be relaxed.

The regulatory body also stated that “fixed-dose combinations are to be used judiciously and only approved and rational fixed-dose combinations are to be prescribed.”
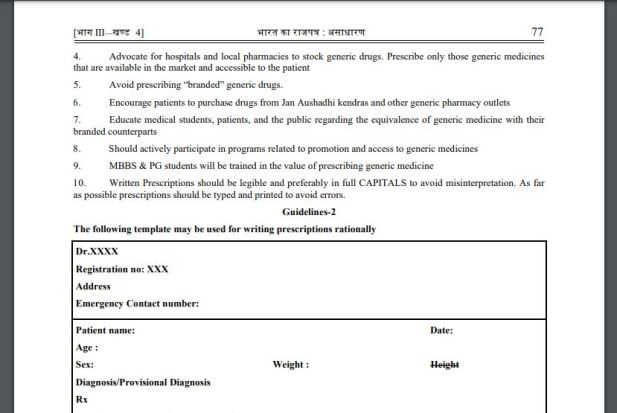
As per the NMC guidelines, the doctors should “advocate” hospitals and local pharmacies to stock generic drugs. Prescribe only those generic medicines that are available in the market and accessible to the patient.
The guidelines also stated the following:
- Avoid prescribing “branded” generic drugs.
- Encourage patients to purchase drugs from Jan Aushadhi Kendras and other generic pharmacy outlets.
- Educate medical students, patients, and the public regarding the equivalence of generic medicine with their branded counterparts.
- Should actively participate in programs related to promotion and access to generic medicines.
- MBBS & PG students will be trained in the value of prescribing generic medicine.
- Written Prescriptions should be legible and preferably in full CAPITALS to avoid misinterpretation. As far as possible prescriptions should be typed and printed to avoid errors.

Moreover, NMC also shared a template on how to write prescriptions “rationally.”
‘Urgent intervention by the government needed’
According to Nikkhil K Masurkar, CEO, Entod Pharmaceuticals, the National Medical Council’s (NMCs) new regulations to enforce doctors to prescribe only generic medicines is “ill-advised, irrational and almost laughable.”
“The molecules that come under price control under Drugs (Prices Control) Order (DPCO) already have their ceiling prices capped irrespective whether they are generic or branded. This means that the NMCs regulations only applies to medicines not under price control and whose MRPs cannot be controlled irrespective whether branded or generic. For example, a medicine that is not under price control can be priced at any level irrespective of it being branded or generic. So technically the price of a generic medicine can be the same as a branded one if the molecule doesn’t fall under price control, which beats the whole point of patient affordability,” Masurkar told Financial Express.com.
He also said that the words “generic” or “branded” have no relevance when it comes to DCPO price control as it’s the molecule that is controlled and not its brand or generic status.
“…Will this new regulation help to improve patient affordability? Absolutely not! Infact new generic medicines will be launched with prices as high as their branded counterparts and patients will end up spending the same, if not more. The current DPCO price control system and the NLEM list is more than sufficient to improve patient affordability, and doesn’t even necessitate doctors to prescribe generically. Either the NMC didn’t quite understand the provisions of the Drug Price Control Order or they were just seriously misguided,” he said.
He also said that the IMA did oppose this thankfully but even their argument missed the crucial points above.
“In fact their argument was as bizarre as the regulation itself when they stated that generic drugs would affect medicine quality and patient safety without shedding light on the fact that the same standards of quality, efficacy and safety apply for all medicines in India- be it generic or branded,” he added.
Such an irrational and misguided regulation calls for a wider consultation and serious and urgent intervention by the government,’ he said.
‘This approach compromises the health of the general public’
Dr. Pranesh Raman, a General Physician told Financial Express.com that the practice of prescribing generic medicine for every ailment lacks scientific grounding.
“Unlike ethical companies that invest in comprehensive research and product monitoring, generic manufacturers often copy molecules without adequate oversight. If generic drugs result in unfavourable outcomes, the burden typically falls on doctors, rather than pharmacists, the government, or regulatory bodies like the NMC. This dynamic places doctors in a precarious position,” Dr. Raman said.
Consider a scenario where a doctor prescribes “paracetamol” for fever without specifying a brand name, as per NMC guidelines. Subsequently, the patient acquires paracetamol from a pharmacy, but the choice of manufacturer remains arbitrary, he maintained.
“Notably, the pharmacy isn’t held accountable if the patient’s condition doesn’t improve, whereas the doctor shoulders this responsibility. This approach compromises the health of the general public in favour of cost-cutting measures, potentially leading to issues like medication resistance, incorrect prescriptions, and strained doctor-patient relationships,” he told Financial Express.com.
A more logical approach would involve government efforts in promoting research and development-driven generic medicines, addressing these critical concerns while striving to minimize out-of-pocket expenses for patients, he said.
According to Sandeep D’Souza, Chief of Medical Services, Sakra World Hospital, Bengaluru, there is no doubt that generic medicines are cheaper than branded ones, which brings down the overall cost of medical treatment, but there are a couple of concerns when it comes to generic medicines:
- Lack of quality of generic medicines: Generic drugs are substandard. They simply lack the quality and efficacy of branded drugs. On the other hand, branded drugs are researched, tested, and quality controlled. Pharmaceutical companies that own the brands, ensure they are tested, and quality controlled for desired efficacy.
- Lack of adequate supply: Local pharmacies won’t have stock of generic medicines. They will not keep generic medicines as the margins are very small. They rather keep branded medicines which make better business sense.
- Giving too much power to Pharmacists: When generic drugs are in short supply or not available, the pharmacist will give a branded substitute which he likes, which may be the most expensive one. Besides, he may not know which is good for the patient.
“So, at the end, you won’t get the generic drug, and you will end up with the most expensive brand. The doctor-patient relationship is sacrosanct, and the doctor knows each patient and how each drug will behave with each patient. So, a blanket rule calling for generic drug use for all cases may not be in the best interest of the patient. At the end, there may be a role for generic medicines but not until their quality can be trusted by doctors and patients alike,” D’Souza told Financial Express.com.
Meanwhile, Dr Ashok Shukla, President, Association of Medical Consultants, Mumbai said that the debate over branded drugs vs generic drugs is an ongoing conversation with differing opinions from doctors, pharmacists, medical bodies, and other members of the medical fraternity.
“Under the new NMC regulation, prescribing generic drugs has been mandated in lieu of reducing healthcare costs thus making it affordable and accessible to the people of the country. However, providing quality healthcare is also of prime importance in addition to accessibility and affordability. Generic drugs are no doubt comparable to branded drugs, but its quality and performance has to be assured too,” Dr. Shukla told Financial Express.com.
He also emphasised that credibility must be lent to the generic drugs for doctors to be comfortable in prescribing them to their patients.
Meanwhile, Dr. Rajiv Chhabra, Chief Paediatric, Artemis Hospitals, Gurugram revealed that the although he has witnessed several shifts in our healthcare sector, but the recent NMC regulation mandating the prescription of generic drugs “resonates” with him.
“Seeing firsthand how families, including mine, grapple with medical expenses, I genuinely appreciate the cost-effectiveness of generic medicines. They don’t just lighten the financial load but ensure that many of our fellow citizens, especially those with limited means, can consistently access the healthcare they need. Besides affordability, I’ve always believed in the principle of wider availability, and it’s heartening to see the regulation champion that…,” Dr. Chhabra told Financial Express.com.
However, he also acknowledges the concerns that others healthcare professionals are raising. “I would like to summarise by saying that while I’m optimistic about the NMC regulation and the sweeping changes it promises, I can’t help but feel that its success lies in its execution,” he pointed out.
‘Generic medications are substantially more economical’
Aniruddha Sen, Co-Founder, Kenko Health maintains that as the doctors are now mandated to prescribe generic medications for patients, a notable upswing in the utilization of generic drugs, particularly in tier-2 and tier-3 cities is anticipated.
The pharmaceutical market in India is of colossal proportions, projected to soar to $130 billion by 2030, and globally ranks third in terms of production. Yet, the issue of affordability remains a big concern, with room for enhancing accessibility to pharmaceutical products across the populace. This affordability disparity also contributes significantly to the higher costs of preventive healthcare,” Sen told Financial Express.com.
He believes that the NMC’s new regulations are likely to change this narrative.
This shift will also mitigate the biases on medical professionals’ part to exclusively prescribe drugs from particular brands. This will make healthcare more affordable and accessible to citizens on a large scale, he added.