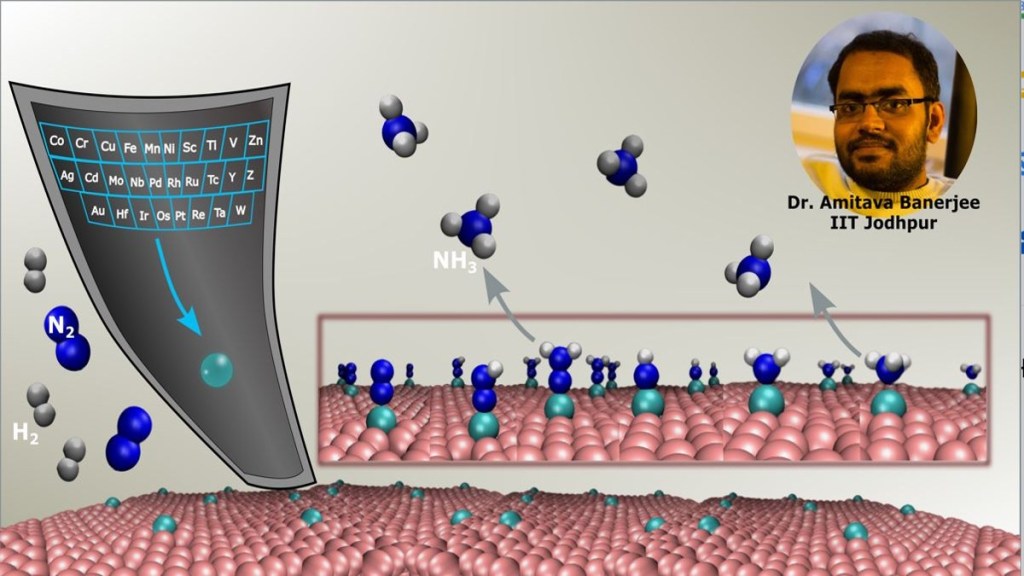
Indian Institute of Technology (IIT) Jodhpur researcher Amitava Banerjee along with his team has identified single-atom catalysts for Nitrogen Reduction Reaction (NRR) required for the synthesis of ‘Green Ammonia’, as per the press statement.
At present, the conventional process of synthesising green ammonia has an enormous carbon footprint, as it emits 3% of global carbon dioxide and consumes 2% of the world’s total energy production. Electrochemical synthesis route could be a vital choice for its synthesis, where Nitrogen Reduction Reaction (NRR) is one of the hardest reactions to carry out due to the strong N-N triple bond coupled with poor nitrogen adsorption on many catalysts and presence of competitive hydrogen evolution reaction. So, the researchers focused on electrochemical NRR in order to synthesise ‘Green Ammonia’, as per the press release.
Currently, the team is focusing on the design of electrocatalysts for green ammonia and green urea production. Both will have less or negligible carbon footprint compared to existing conventional processes. Urea is a vital compound for societal development and the high presence of 46% nitrogen (by weight) makes it a major player of the fertiliser industry.
Ammonia is also one of the key elements in the fertiliser industry as well as in paper, textile and rubber industries. Moreover, it could be a potential carrier for hydrogen for easy transportation and storage of the fuel, which may speed up the hydrogen economy in our country by utilising the existing ammonia pipelines.
“The recent surge in interest in the electrochemical synthesis of NH₃ has highlighted the inadequacy of Nitrogen Reduction Reaction (NRR) catalysts. So, our group’s primary objective is to computationally design the NRR catalysts and provide insight for the experimentally obtained NRR catalytic mechanism,” Amitava Banerjee, assistant professor, Department of Metallurgical and Materials Engineering, IIT Jodhpur, said.
As an extension of the ongoing search for NRR catalysts, the group has also discovered in collaboration with a catalytic mechanism involving two different transition metals, in collaboration with Dr. Ghorai’s group of Ramakrishna Mission Vidyamanidira, Belur Math. This mechanism efficiently activates the nitrogen-nitrogen (N-N) triple bond and promotes the formation of carbon-nitrogen (C-N) bonds, ultimately leading to the production of environmentally friendly urea, it added.