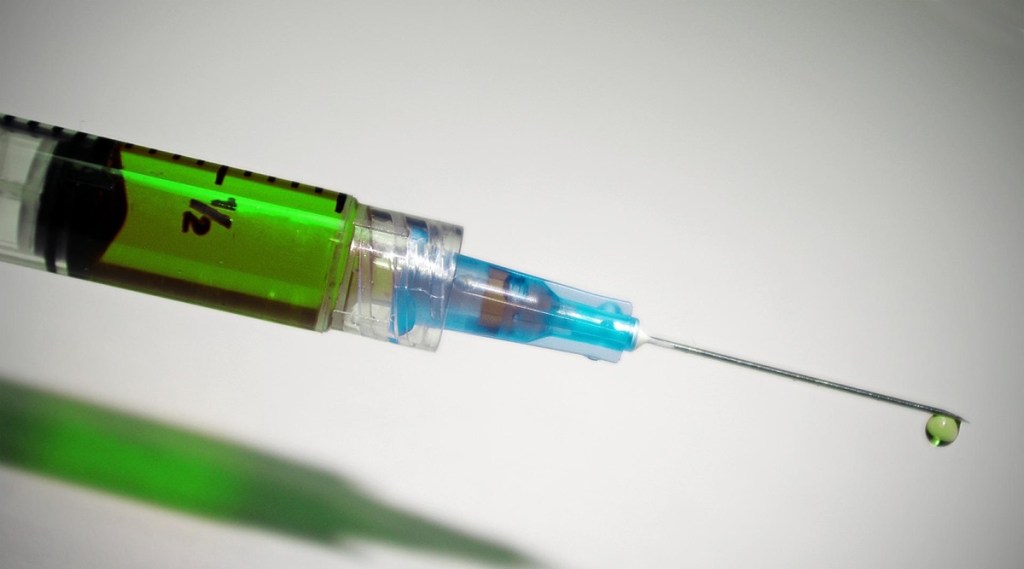
The U.S. Food and Drug Administration (USFDA) on Friday approved Ixchiq, the first chikungunya vaccine. Ixchiq is approved for individuals 18 years of age and older who are at increased risk of exposure to chikungunya virus. The FDA granted approval of Ixchiq to Valneva Austria GmbH.
The chikungunya virus is primarily transmitted to people through the bite of an infected mosquito. Chikungunya is an emerging global health threat with at least 5 million cases of chikungunya virus infection reported during the past 15 years.
The highest risk of infection is in tropical and subtropical regions of Africa, Southeast Asia, and parts of the Americas where chikungunya virus-carrying mosquitos are endemic. However, chikungunya virus has spread to new geographical areas causing a rise in global prevalence of the disease.
The most common symptoms of chikungunya include fever and joint pain. Other symptoms may include a rash, headache, and muscle pain. Some individuals may experience debilitating joint pain that persists for months or even years. Treatment includes rest, fluids, and over-the-counter medications for pain and fever.
“Infection with chikungunya virus can lead to severe disease and prolonged health problems, particularly for older adults and individuals with underlying medical conditions,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “Today’s approval addresses an unmet medical need and is an important advancement in the prevention of a potentially debilitating disease with limited treatment options.”
Ixchiq is administered as a single dose by injection into the muscle. It contains a live, weakened version of the chikungunya virus and may cause symptoms in the vaccine recipient similar to those experienced by people who have chikungunya disease.
The FDA is requiring the company to conduct a postmarketing study to assess the serious risk of severe chikungunya-like adverse reactions following administration of Ixchiq.
“Ixchiq was approved using the Accelerated Approval pathway. Accelerated approval allows the FDA to approve certain products for serious or life-threatening conditions based on evidence of a product’s effectiveness that is reasonably likely to predict clinical benefit. In the FDA’s evaluation of Ixchiq for accelerated approval, evidence of effectiveness is based on immune response data in clinical trial participants. As a condition for approval for Ixchiq, the FDA is requiring confirmatory clinical studies to be conducted to verify clinical benefit,” the company said in a statement.
Ixchiq was granted Fast Track and Breakthrough Therapy designations and the application was granted Priority Review.
In addition, the FDA awarded the manufacturer of Ixchiq a tropical disease priority review voucher, under a provision included in the Food and Drug Administration Amendments Act of 2007.