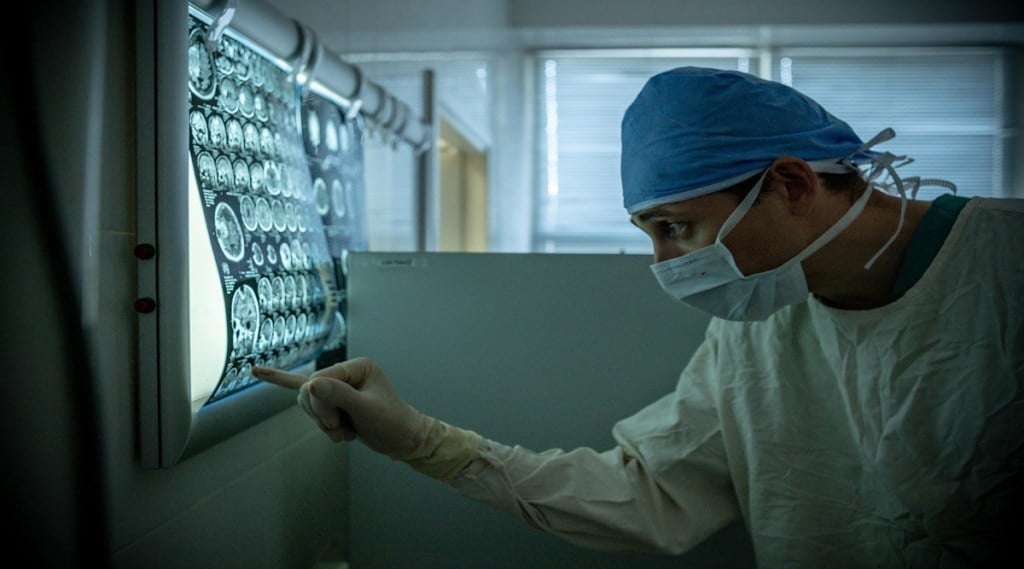
For years, scientists have been struggling to understand and find a cure for Alzheimer’s disease. Now, the results from significant trial results show that the wait might be over soon.
According to the trial results, the drug, Donanemab, was found to slow “clinical decline” by up to 35 percent. Developed by US pharmaceutical company Eli Lilly, it is an anti-amyloid monoclonal antibody therapy specifically designed to remove the accumulation of beta-amyloid protein which is a characteristic of Alzheimer’s disease.
The researchers claim that with this drug patients can easily perform their day-to-day activities shopping, housekeeping, financial management, and medication adherence.
After the results became public, Alzheimer’s Research UK said “we’re entering a new era” where the disease “could become treatable”. Meanwhile, scientists have published the final results of a trial, known as TRAILBLAZER ALZ-2, examining the safety and efficacy of the drug.
Reportedly, if the drug gets approved by the USFDA, it will become the third medication available to treat the condition in the US.
The drug is administered through an intravenous infusion once every four weeks. The patients are required regular brain scans to monitor potential side effects, such as brain swelling and bleeding.
Although these side effects usually resolve on their own, in rare cases they can be fatal. According to media reports, the trial reported there were severe side effects that came with the treatment. Consequently, the medication is mostly effective for those in the early stages of the disease.
Reportedly, more than 6 percent of the 860 patients who received donanemab infusions experienced symptoms associated with brain bleeds and swelling, such as confusion, headaches and seizures.
Interestingly, the drug is similar to two approved Alzheimer’s treatments: aducanumab, which was approved in 2021, and lecanemab, which was approved in January this year.
The results of the trial were published in the Journal of the American Medical Association.