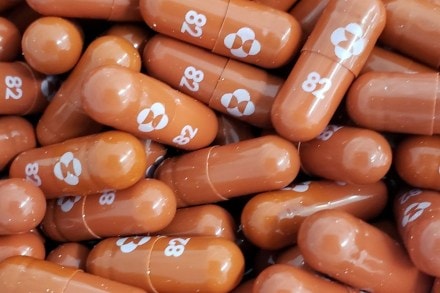
Pharma major Merck has announced its plans to seek emergency use authorisation (EUA) from the USFDA for using Molnupiravir as a antiviral medicine for Covid-19. The company also plans to submit marketing applications to regulatory agencies worldwide. If authorised, Molnupiravir could become the oral drug for treating Covid-19. The Molnupiravir drug is also undergoing trials in India.
The Indian companies are looking at the implications of the USFDA approval on the ongoing trials and timeline in India. Merck, known as MSD India, has tied up with eight Indian companies to manufacture the drug in India. In April this year, the company had entered into voluntary licensing agreements with generic manufacturers in India to supply its investigational oral antiviral drug candidate, Molnupiravir.
Cipla, Dr Reddy’s Laboratories, Sun Pharma, Hetero Labs and Emcure Pharmaceuticals have signed deals with MSD. Torrent Pharma, Aurobindo Pharma and Viatris Pharma, too, inked deals with Merck. These agreements were expected to accelerate the availability of Molnupiravir in India and in 100 other low- and middle-income countries after regulatory approvals.
Cipla, Dr Reddy’s, Emcure, Sun and Torrent are collaborating with MSD for clinical trials of the Monupiravir in India.
On completion of the trials, each company would independently approach the Indian regulator for approval to manufacture and supply the Molnupiravir for treatment of Covid in India. Divi’s Labs will be supplying active pharmaceutical ingredients for Molnupiravir to Merck’s partners in India.