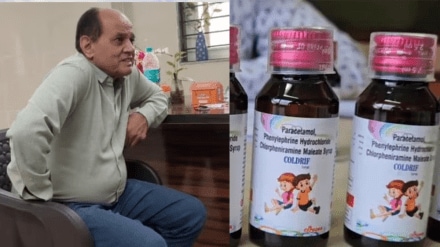
After the arrest of Madhya Pradesh’s senior paediatrician Dr Praveen Soni, in connection with the 16 deaths allegedly linked to Coldrif cough syrup, the Indian Medical Association (IMA) has come out in his defence. The doctors’ association claims Dr Soni is being unfairly targeted and should not be solely held responsible for the tragedy.
While questioning the clean chit awarded to the pharma company, the association has said that it would continue to push for Dr Soni’s release, according to a report by India Today. Kalpana Shukla from the association has warned that if Dr Soni is not released soon, the doctors will go on an indefinite strike.
FIR, suspension and arrest
Dr Soni was arrested on Saturday after police lodged an FIR against him and the operators of Sresun Pharmaceuticals, the manufacturers of Coldrif cough syrup, under several sections of the BNS and the Drugs and Cosmetics Act. The FIR, which was filed on the complaint of Dr Ankit Sahlam, Block Medical Officer of Parasia Community Health Centre, mentioned that the cough syrup was contaminated.
According to Dr Sahlam’s complaint, several children who were treated by Dr Soni for fever and cough later developed symptoms of acute kidney failure.
Following this, the Madhya Pradesh government suspended Dr Soni with immediate effect for alleged negligence in treating the affected children. The action was taken under the direction of Chief Minister Mohan Yadav.
Dr Soni defended himself
According to several reports, Dr Soni said that he has been prescribing this cough syrup for several years now, and he is not the only one prescribing this.
“This syrup is not a one-day treatment. I have been prescribing medicines from this company for over ten years,” Dr Soni told India Today hours before his arrest.
He added, “It’s wrong to suggest that a primary doctor decides on the formulation. We receive ready-to-use, sealed medicines.”
Lab report confirms toxic susbstance
A laboratory report confirmed that 48.6% Diethylene Glycol (DEG), a toxic compound known to cause kidney failure and death if consumed, was found in the syrup.
Following the significant number of deaths, 19 in total, which were linked to Coldrif, several states have banned the cough syrup. Madhya Pradesh, Rajasthan, Tamil Nadu, Kerala and Uttar Pradesh have banned the cough syrup in their states. Karnataka and Telangana have also directed officials to remain vigilant and sensitise people about the same.
Amid all this, teams have left for Chhindwara and Tamil Nadu to meet local authorities and to assess the situation. A special investigation team (SIT) has also been constituted to trace the supply chain of the toxic batch and identify accountability at the manufacturing level.